Abstract
We examine the extent to which intrinsic and extrinsic factors influence reproductive phenology in male bats at the population level. Using data from thirteen breeding seasons (2006–2018), encompassing the reproductive histories of 1546 Myotis daubentonii and 530 M. nattereri males, we compare rates of sexual maturation and the temporal distribution of phases of spermatogenesis between juvenile (born that season) and adult (born in previous seasons) males. We found that (i) higher proportions of M. daubentonii (50.81%) than M. nattereri (12.85%) became sexually mature as juveniles, (ii) the proportion of juveniles in reproductive condition per annum was influenced by spring weather conditions, (iii) in both species males that reached puberty as juveniles had higher body mass, on average, than immature juveniles, (iv) older males (aged ≥4 years old) commenced spermatogenesis earlier than young adult males (aged 1–3 years old), whilst juveniles that commenced spermatogenesis did so later in the year than adults, in both species, and (v) M. daubentonii commenced and completed spermatogenesis earlier than M. nattereri in the equivalent age class. Our findings suggest that selection pressure exists for early mating readiness and synchronisation with female receptivity.
Similar content being viewed by others
Introduction
Seasonal variation in climate and resource availability (e.g. prey abundance) imposes energetic constraints on the timing of reproduction for many organisms in both temperate and tropical environments1,2. Previous studies have confirmed the existence of seasonal breeding cycles in bats from several genera, occupying diverse habitats and ecological niches3. Flexibility in the frequency, timing, and characteristics (e.g. whether or not prolonged sperm storage occurs in either sex) of breeding cycles are even known to occur across the range of some widely distributed species in response to local conditions4. At temperate latitudes the reproductive cycle of hibernating bat species is interrupted by winter5. The mating season commences in late summer and mating activity peaks during autumn6, but copulation also occurs (during periods of arousal for one or both parties) throughout hibernation7,8, and some mating effort may resume during spring (to varying extents in different species)9.
Prolonged sperm storage can occur in bats of either sex, enabling separation of spermatogenesis and mating effort for males5, and an interval (often of several months duration) between copulation and conception in breeding females10. Sperm storage over winter enables inseminated females to commence gestation as early as possible on emergence from hibernation (i.e. without using limited energy reserves or spending time on mating activities in spring)11. Earlier parturition, given favourable conditions, can increase reproductive success and survival rates of both mother and offspring12,13,14. Prolonged storage of viable sperm in male bats has enabled a unique asynchrony to develop between spermatogenesis and the functioning of accessory sex organs and libido5. Spermatogenesis commences in spring in adult males in temperate zones and is completed during the peak of food availability and favourable climatic conditions during summer. Sperm then transfer to the caudae epididymides and mating readiness occurs in synchrony with females from late summer onwards5,15,16.
Testicular size and manifest engorged epididymides correspond with histological examination of seminiferous tubules during spermatogenesis, and of stored epididymal spermatozoa, respectively5. External physical examination and visual assessment of testes and epididymides enlargement therefore provide robust and less invasive measures of reproductive condition for monitoring seasonal patterns of reproductive condition in wild populations6.
Juvenile males can achieve sexual maturity during the season of their birth17,18,19,20,21. Birth timing is indirectly influenced by spring weather conditions affecting the duration of gestation and parturition dates14,22. Whether the proportion of juvenile males becoming sexually mature varies between breeding seasons has not previously been ascertained.
Previous studies on the phenology of reproductive condition in wild male bats have focused on opportunistic sampling during autumn swarming activities at hibernation sites6. To our knowledge this is the first study with repeat sampling of known individuals from their summer habitat, following male juveniles from volancy through to sexual maturation, including comparison of reproductive phenology between juveniles, young adults (aged 1–3 years old) and older males (aged ≥4 years old).
Based on our observations that M. daubentonii are born earlier than M. nattereri within our study system14, we predict that:
- (i)
higher proportions of M. daubentonii than M. nattereri males will reach sexual maturity as juveniles,
- (ii)
higher proportions of juvenile males will come into reproductive condition during breeding seasons with favourable spring weather conditions (when parturition dates are earlier),
- (iii)
spermatogenesis will commence and be completed earlier in M. daubentonii compared to M. nattereri (we postulate this enables males to synchronise with the reproductive phenology of females, with mating activities commencing as soon as females become receptive at the end of the maternity period).
Based on longitudinal observations of changes in male body mass and spermatogenesis with age, we also test whether:
- (iv)
juveniles achieving puberty have higher body mass, on average, than immature juveniles, and adult non-breeders have lower body mass on average compared to adults that undergo spermatogenesis.
- (v)
spermatogenesis occurs earlier in older males (aged ≥4 years old) compared to young adult males (aged 1–3 years old), and juveniles that reach puberty commence spermatogenesis later than adult males.
Results
Age at sexual maturity varies between species
As predicted, greater proportions of juvenile male M. daubentonii than M. nattereri became sexually mature during their birth year (at approximately 2–5 months old) (see Fig. 1a and Model I in Table 1). 282/555 (50.81%) juvenile male M. daubentonii reached puberty (reproductive condition B, C or D), the other 273/555 (49.19%) were still sexually immature (non-reproductive condition A) during September or October in the year of their birth. In marked contrast, only 32/249 (12.85%) juvenile male M. nattereri were found in reproductive condition. Even if ‘unknown’ (minimum age = 0) M. nattereri males are included (which is likely to produce an overestimate as this category comprises a mixture of advanced juveniles and young adults) only 46/259 (17.76%) male M. nattereri of juvenile and ‘unknown’ age status were found in reproductive condition in the year they were first encountered.
From one year of age onwards, almost all adult males (over 85% of M. nattereri, and more than 98% of M. daubentonii, respectively) came into reproductive condition each season (see Fig. 1a and Supplementary Table S1).
Spring weather conditions affect the proportion of juvenile males in reproductive condition
ASFC (April Suitable Foraging Conditions; our index of spring weather conditions) was retained during model selection as a significant predictor of the proportion of juvenile males becoming sexually mature each season (see Fig. 1b and Model I in Table 1). For M. daubentonii the proportion of juvenile males in reproductive condition in 2011 (the breeding season with the highest ASFC, and the earliest parturition dates, in our dataset) was 46/63 (73.02%), compared to only 7/43 (16.28%) in 2012 (the breeding season with the lowest ASFC, and latest parturition dates, in our dataset). Similarly, the majority of male M. nattereri juveniles that reached puberty were encountered during the five years with the highest ASFC values in our dataset, and no juvenile male M. nattereri were recorded as reaching sexual maturity during the four years with the lowest ASFC values in our dataset (see Fig. 2f).
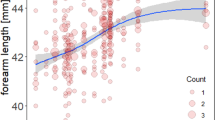
Body mass (g) of male Myotis daubentonii (circles) and M. nattereri (triangles) plotted for adult males (left panels; a, c and e) and juvenile males (right panels; b, d and f) against: DOTY (day of the year) (a,b), FA (forearm size (mm)) (c,d), and ASFC (an index of spring weather conditions) (e,f). Mean body mass is plotted (filled blue circles and solid blue lines for M. daubentonii, filled green triangles and solid green lines for M. nattereri, achieving reproductive condition that season. Red open symbols and dashed lines for non-breeders that season in both species), fit by loess (a,b) or linear regression (c–f), shaded areas (blue for M. daubentonii, and green for M. nattereri) represent 95% confidence intervals.
Body mass varies with age and reproductive condition
Juvenile non-breeders had lower body mass, on average, than males that became sexually mature as juveniles (see Fig. 2b and Model II in Table 1). Adult males that achieved reproductive condition were significantly heavier on average than adult non-breeders, and adults were significantly heavier on average than juveniles in both species (see Fig. 2 and Model II in Table 1). Body mass was also influenced by day of the year (DOTY), and interactions between DOTY and species (SPP), age class and reproductive condition (ACL_RC), and ASFC (see Fig. 2, and Model II in Table 1, and Supplementary Table S3). Forearm (FA) was retained in the top-ranked model as a significant predictor of male body mass (see Model II in Table 1). Male M. daubentonii are, on average, significantly heavier (but have smaller forearms) than male M. nattereri (see Fig. 2c,d).
Phenology of reproductive condition in relation to species and age
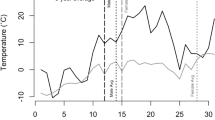
The onset of spermatogenesis occurred earlier in male M. daubentonii compared to M. nattereri of equivalent age class (see Fig. 3 and Model III in Table 1). In both species the onset of spermatogenesis was advanced in older males (age ≥4 years old) compared to young adult males (aged 1–3 years old). Spermatogenesis occurred significantly later in juveniles than in adults in both species (see Fig. 3 and Model III in Table 1). An interaction between DOTY and ASFC was retained in the top-ranked model indicating that reproductive phenology was advanced during breeding seasons with a higher ASFC (see Model III in Table 1 and Supplementary Table S4).
Phenology of phases of spermatogenesis (A > B > C > D) (see methods and supplementary Fig. S2), in male M. nattereri (green) and M. daubentonii (blue) per age class. Lines A–A, B–B, C–C, and D–D, indicate mean date for that reproductive phase per species and age class. The y-axis corresponds to count data (sample size). Female reproductive phenology is also presented (not to scale on y-axis) for temporal comparison.
Discussion
As predicted, rates of sexual maturation in juvenile males, and the reproductive phenology of adult males, were found to correspond with the phenology of breeding females within our study system14. Earlier parturition dates in M. daubentonii compared to M. nattereri corresponded with higher proportions of juvenile male M. daubentonii than M. nattereri reaching puberty at 2–5 months old. During breeding seasons with favourable spring weather conditions, when parturition dates were earlier than average, higher proportions of juvenile males became sexually mature in both species. Males in breeding condition had higher body mass, on average, compared to males in the equivalent age class that were non-reproductive. Adult male M. daubentonii commenced spermatogenesis earlier than M. nattereri, and in both species older males were found to be more advanced in their reproductive condition compared to young adult males.
Adult M. daubentonii males typically commence spermatogenesis during June and July then leave our study system (summer habitat) when they become sexually active (with sperm stored in the epididymides indicating mating readiness6,21), presumably to participate in autumn swarming. Mating at swarming sites may maximise their breeding potential through copulation with numerous sexual partners and outbreeding with non-related females from different colonies drawn from a large catchment area16. In contrast, M. nattereri adult males typically commence spermatogenesis during July and August then leave their summer habitat (presumably to migrate to autumn swarming sites23,24,25) during September. These observations fit well with the temporal distribution of swarming activity recorded in these species, with the peak in M. daubentonii activity at swarming sites occurring during August, and the peak in M. nattereri activity at swarming sites occurring during September25,26,27,28. The difference in timing of spermatogenesis between species also corresponds with the breeding phenology of females, with independent juveniles and post-lactating M. daubentonii being recorded from early July onwards, whilst volant juveniles and post-lactating M. nattereri are recorded from late July onwards in our study system14.
Adult males in breeding condition (with sperm in their epididymides) have been found sharing day roosts with potentially receptive females from late July (M. daubentonii) and mid-August (M. nattereri) onwards on numerous occasions, suggesting that summer resident males may be responsible for the paternity of some juveniles within our study system16,29. In the absence of behavioural data on mating activities, or genetic data to assign paternity, there is currently no data available on whether or not juvenile males that become sexually mature are mating or fathering offspring.
Potential sampling bias exists within our dataset as males commencing spermatogenesis were recorded from June onwards whilst non-breeders were determined only during September or October (males encountered in non-reproductive condition during June to August may have commenced spermatogenesis later that breeding season when not observed). However, there is also a counter bias whereby males found to have commenced spermatogenesis during June to August are rarely encountered during September and October, probably due to their migration to autumn swarming and winter hibernation sites. The adult males that are recorded during September and October are more likely to be non-breeders, or males that commenced spermatogenesis later than average, which counter-balances to some extent the longer time period over which males in reproductive condition are recorded. From mid-September onwards the majority of males encountered within this summer habitat are juveniles (see Fig. 3).
Spermatogenesis may be triggered by environmental or endogenous cues30, and selection pressure may operate to synchronise the energy expenditure associated with spermatogenesis to seasonal peaks in food availability2,18, rather than there being any direct synchronisation with female reproductive cycles (except for juvenile males which are likely to be directly influenced by parturition dates and consequent developmental and maturation rates). Nevertheless, despite the temporal separation between spermatogenesis and mating that is possible in the reproductive cycle of temperate bats due to prolonged sperm storage5,10 it seems likely that a selective advantage does exist for males to be ready to mate soon after females have completed lactation. Mating within day roosts in the summer habitat16, and peak autumn swarming activities25,26,27,28, both occur within a short period that is post-maternity and pre-hibernation for our two study species. The timing of mating readiness in males and receptivity in females differs between these sympatric congeners but is highly synchronised within each species. The ‘early male advantage’ hypothesis6,31 is also supported by our empirical evidence that older males (which are in better body condition, see Supplementary Fig. S1), commence spermatogenesis and are in breeding condition earlier than young adult or juvenile males.
Whether or not selection pressure exists for early maturation in juvenile males remains uncertain. The fact males that undergo spermatogenesis as juveniles have higher body mass, on average, than juveniles which remain immature, suggests early maturation is not a costly trait given suitable conditions (e.g. early birth timing which allows sufficient time for development and puberty prior to hibernation). The proportion of juvenile males mating successfully remains unknown. Even low incidence of paternity amongst juveniles could significantly contribute to lifetime reproductive success at the individual level, and affect gene flow at the population level, as survival rates are lower in juveniles than adults32.
Although forearm size was a significant predictor of body mass, using a body condition index to account for variation in body size based on forearm measurements would not necessarily provide a better predictor of fat mass than uncorrected body mass33. Body mass has been empirically proven to be significantly positively correlated with fat mass in Myotis lucifugus31, a Nearctic species with morphological similarities to the Palearctic M. daubentonii34. Previous studies31 have also noted a small proportion of exceedingly heavy (>10.0 g) adult males. We observe that such heavyweights are predominantly older male M. daubentonii, especially during mid-August to mid-September (see Supplementary Fig. S1). Due to the energetic constraints imposed by flight it is deemed unlikely that such heavyweight males participate in swarming for their main mating strategy (heavyweight males are indeed underrepresented in swarming captures, DML unpublished data). It therefore seems likely that several, not entirely mutually exclusive, and possibly varying with age and body condition, mating strategies exist. These include fathering offspring with females from maternity (even natal) colonies resident in the same summer habitat16,29, autumn swarming16,24, and a third possibility, that heavyweight males are able to sustain more frequent arousals and copulations during hibernation8. Our findings correspond with previous observations19 that although male bats are capable of reaching sexual maturity at 2–5 months of age, full maturation (i.e. earlier onset of spermatogenesis, and possibly a shift in mating strategies, with increasing age) is a process that takes several years. Demonstrating that, as small-sized but long-lived mammals, bats exhibit unusual combinations of life-history traits35.
Our results also indicate that using sexual immaturity as a determining factor for distinguishing juvenile males during autumn is not valid for all medium-sized temperate Myotis species6,7,8,31,36. Altitudinal and latitudinal gradients, however, may contribute to geographical variation in the proportions of juveniles reaching puberty due to variation in the timing of parturition (in relation to severity of spring climate) and therefore time available to complete development prior to hibernation20,31,37.
Many temperate zone bat species exhibit adaptations to incorporate hibernation within their reproductive cycle, including an extended mating season lasting from late summer into autumn, through winter, and even into spring6,9,16,31. In many species both sexes are capable of storing sperm for up to several months4, which enables inseminated females to ovulate and commence gestation with minimal energy expenditure in spring10,11. These adaptations may make hibernating bats less sensitive to mismatches in phenology and climatic variation than other taxa where all reproductive stages occur in rapid succession within a restricted period during which breeding success is dependent on sustained favourable conditions1.
Methods
Data collection
Bats were encountered in day roosts within SchweglerTM woodcrete boxes distributed across our study site, Wytham Woods (51°77′N, 1°33′W) or caught at night in mist nets and harp traps in the same locality. All catching, handling and ringing of bats was carried out under project licence from Natural England (2018-36143-SCI-SCI and preceding licences). All bats were returned to their roost, or released at the site of capture, as soon as possible following examination.
Assessment of reproductive condition
The presence or absence of enlarged testes and distended caudae epididymides (swelling being indicative of spermatogenesis and sperm storage respectively), was recorded based on visual assessment by experienced surveyors during manual handling of bats.
Males were classified into four categories of reproductive condition38: (A) non-reproductive (testes not swollen, epididymides not distended), (B) testes swollen, epididymides not distended, (C) testes swollen, epididymides distended, and D) testes not swollen, epididymides distended, see Supplementary Fig. S2). In male bats there is a progression from A > B > C > D (a process taking 1–2 months per individual once commenced, based on 271 within-season recaptures of 105 males that were observed to progress through stages of spermatogenesis) per breeding season in all reproductively active males.
Assessment of whether or not individual males were in reproductive condition each season was based on any encounter where swollen testes and/or epididymides (reproductive condition B, C or D) were observed during June to October. Encounters with adult males with distended epididymides but without enlarged testes in spring (n = 27 bats on 30 occasions during April and May) were excluded in an attempt to record the temporal occurrence of spermatogenesis per annum (i.e. enlargement of the testes due to spermatogenesis prior to distension of the caudae epididymides due to sperm storage), and not vestiges of the previous breeding season. Only males encountered during September or October in non-reproductive condition (A) were classified as non-breeders that season. Individuals encountered only during April to August in non-reproductive condition (A) were classed as ‘unknown’ breeding status for that season, as they might still have come into reproductive condition later whilst not observed. Some individuals found in non-reproductive condition (A) during August were recaptured in reproductive condition (B or C) during September.
Age assessment
Males were classified as juvenile (actual age = 0) based on unfused finger joints39 or a suite of secondary characteristics indicative of bats born that season, including ‘fresh’ wing membranes, grey pelage, dense ‘chinspot’, dark tunica vaginalis, and light weight18,32,40,41, once the epiphyseal gaps were fully ossified. Adult males (minimum age = 1) were identified on first capture based on timing (encountered early in the season before juveniles were full-grown), evidence of prior reproduction such as elongation of the caudae epididymides or unpigmented tunica vaginalis, and colouration or weight15. Bats of indeterminate age, possessing an ambiguous mixture of juvenile and adult characteristics, were classified as ‘unknown’ (minimum age = 0) when ringed. The majority of data for adult males (2324/2693 = 86.30% of M. daubentonii encounters, and 807/887 = 90.98% of M. nattereri encounters) were derived from confirmed adults (i.e. ringed individuals recaptured 1–12 years post-ringing).
The proportion of males in reproductive condition per age class was based only on individuals of known (actual) age for age classes 0, 1, 2 and 3 (ringed as juveniles and recaptured 0–3 years later), age class ≥4 included bats of known and minimum age (ringed at least four years before as a juvenile or ‘unknown’, or at least three years earlier as an adult).
Biometric measurements
Forearm length was measured to the nearest 0.1 mm using dial calipers (n = 3779, 1–24 measurements per male). Body mass was recorded to the nearest 0.1 g using a spring balance (n = 3598, 1–22 measurements per male). Body mass measurements obtained from males captured at night were excluded from our analyses as during foraging bouts substantial weight gain can occur due to ingested prey.
Data Analysis
Model I: Reproductive condition in juvenile males
The proportion of juvenile males reaching puberty was treated as a binary response variable (Y = found in reproductive condition (B, C or D) as a juvenile, N = found in non-reproductive condition (A) during September or October in the year of their birth) in logistic models, using the ‘glmer’ function in package lme442 in R version 3.5.343, with species (SPP = Myotis daubentonii, abbreviated to Md, or M. nattereri, abbreviated to Mn) as a categorical explanatory variable, and spring weather conditions during their birth year (ASFC which is a proxy for breeding phenology and birth timing14) as a continuous explanatory variable. We also tested for an interaction between SPP:ASFC in case the effect of spring phenology varied between species (details of model selection are presented in Supplementary Table S2). Year as a factor (fYR) was included as a random intercept effect to provide the correct level of replication.
Model II: Does body mass vary with age and reproductive condition?
Due to the temporal spread of body mass (g) and reproductive condition (A, B, C and D) data throughout the summer survey season (see Figs. 2a,b and 3) it was not possible to incorporate weight as an explanatory variable in an analysis of reproductive condition as determining cause and effect between body mass and reproductive condition was problematic (i.e. the weight of males in non-reproductive condition A may influence the likelihood and timing of spermatogenesis, but the body mass of males in reproductive condition B and C may have been influenced by energy expenditure during spermatogenesis). We therefore used body mass as the response variable in linear mixed models, using the ‘lmer’ function in R package lme442, with SPP, and age class and breeding status (ACL_BS with four categories; J_NB = juvenile non-breeder, J_RC = juvenile achieving puberty, A_NB = adult non-breeder that season, and A_RC = adult in reproductive condition that season) as categorical explanatory variables, and day of the year (DOTY), ASFC and forearm size (FA) as continuous explanatory variables. We also tested for interactions between predictor variables in case the effect of ACL_BS varied between SPP, or in case the effects of DOTY and ASFC varied between SPP, ACL_BS, or in relation to FA, an interaction between DOTY and ASFC was also tested (see Supplementary Table S3 for details of model selection). Because these datasets contained repeat measures (individuals were observed on between 1–8 occasions per annum, for 1–13 breeding seasons) both individual (IND = ring no. a unique identifier per individual) and fYR were included as random intercept effects to give the correct level of replication. Based on recent recommendations33 we did not correct body mass for forearm size. Due to visual examination of Fig. 2c,d, however, FA was included as a predictor variable.
Model III: Phenology of reproductive condition in relation to species and age
Cumulative Link Mixed Models (CLMMs) with the progression of reproductive condition (A > B > C > D) on an ordinal scale as our response variable were performed using the ‘clmm’ function in package ordinal44 in R. SPP and age class (ACL with three categories; juveniles, young adults (aged 1–3 years), and older males (aged ≥4 years old), were included as categorical explanatory variables. DOTY and ASFC were included as continuous explanatory variables. We tested for differences in the temporal distribution of reproductive phases by including interaction terms between DOTY, SPP and ACL. We also tested for interactions between ASFC and the other predictor variables (see Supplementary Table S4 for details of model selection). IND and fYR were included as random intercept effects to provide the correct level of replication. Only bats known to have commenced spermatogenesis that breeding season were included in the CLMM dataset.
Model selection
Akaike Information Criterion corrected for small sample size (AICc) and model weights were derived using package AICcmodavg45 in R, and were used to rank model sets (details of model selection are provided in Supplementary Tables S2–S4) in accordance with an Information-Theoretic approach46. Results from the favoured model in each set are presented in Table 1.
Ethical approval
All catching, handling and ringing of bats was carried out under project licence from Natural England (2018-36143-SCI-SCI and preceding licences). These methods were carried out in accordance with relevant international, national and institutional guidelines and regulations.
Data availability
Data is available from the corresponding author on request.
References
Bronson, F. H. Climate change and seasonal reproduction in mammals. Phil. Trans. R. Soc. B 364, 3331–3340 (2009).
Stevenson, T. J. & Ball, G. F. Information theory and the neuropeptidergic regulation of seasonal reproduction in mammals and birds. Proc. R. Soc. B 278, 2477–2485 (2011).
Krutzsch, P. H. Anatomy, Physiology and Cyclicity of the Male Reproductive Tract. In: Reproductive biology of bats. Academic Press. pp 91-156 (2000).
Racey, P. A. & Entwistle, A. C. Life-history and reproductive strategies of bats. In: Reproductive biology of bats. Academic Press. pp 363-414 (2000).
Gustafson, A. W. Male reproductive patterns in hibernating bats. Journal of Reproduction and Fertility 56, 317–33 (1979).
Pfeiffer, B. & Mayer, F. Spermatogenesis, sperm storage and reproductive timing in bats. Journal of Zoology 289, 77–85 (2013).
Thomas, D. W., Brock Fenton, M. & Barclay, R. M. R. Social Behaviour of the Little Brown Bat. Myotis lucifugus. Behavioral Ecology and Sociobiology 6, 129–136 (1979).
Wai-peng, V. & Brock Fenton, M. Nonselective Mating in Little Brown Bats (Myotis lucifugus). Journal of Mammalogy 69(3), 641–645 (1998).
Furmankiewicz, J., Duma, K., Manias, K. & Borowiec, M. Reproductive status and vocalisation in swarming bats indicate a mating function of swarming and an extended mating period in Plecotus auritus. Acta Chiropterologica 15(2), 371–385 (2013).
Racey, P. A. The prolonged storage and survival of spermatozoa in Chiroptera. J. Reprod. Fert 56, 391–402 (1979).
Jonasson, K. A. & Willis, C. K. R. Changes in body condition of hibernating bats support the thrifty female hypothesis and predict consequences for populations with white-nose syndrome. PLoS ONE 6, e21061 (2011).
Ransome, R. D. Population changes of Greater horseshoe bats studied near Bristol over the past twenty-six years. Biological Journal of the Linnean Society 38, 71–82 (1989).
Ransome, R. D. Earlier breeding shortens life in female greater horseshoe bats. Philosophical Transactions of the Royal Society of London, Series B, Biological Sciences 350, 153–161 (1995).
Linton, D. M. & Macdonald, D. W. Spring weather conditions influence breeding phenology and reproductive success in sympatric bat populations. Journal of Animal Ecology 87(4), 1080–1090 (2018).
Entwistle, A. C., Racey, P. A. & Speakman, J. R. The reproductive cycle and determination of sexual maturity in male brown long-eared bats, Plecotus auritus (Chiroptera: Vespertilionidae). Journal of Zoology, London 244, 63–70 (1998).
Angell, R. L., Butlin, R. K. & Altringham, J. D. Sexual Segregation and Flexible Mating Patterns in Temperate Bats. Plos One 8(1), e354194 (2013).
Speakman, J. R. & Racey, P. A. The influence of body condition on sexual development of male Brown long-eared bats (Plecotus auritus) in the wild. Journal of Zoology 210, 515–525 (1986).
Encarnação, J. A., Dietz, M. & Kierdorf, U. Reproductive condition and activity pattern of male Daubenton’s bats (Myotis daubentonii) in the summer habitat. Mammalian Biology 69(3), 163–172 (2004).
Encarnação, J. A., Kierdorf, U., Ekschmitt, K. & Wolters, V. Age-related variation in physical reproductive condition of male Daubenton’s bats (Myotis daubentonii). Journal of Mammalogy 87(1), 93–96 (2006).
Barclay, R. M. R. Variable Variation: Annual and Seasonal Changes in Offspring Sex Ratio in a Bat. Plos One 7(5), e36344 (2012).
Cryan, P. M. et al. Evidence of Late-Summer Mating Readiness and Early Sexual Maturation in Migratory Tree-Roosting Bats Found Dead at Wind Turbines. Plos One 7(10), e47586 (2012).
Racey, P. A. & Swift, S. M. Variations in gestation length in a colony of pipistrelle bats (Pipistrellus pipistrellus) from year to year. Reproduction 61(1), 123–129 (1981).
Parsons, K. N. & Jones, G. Dispersion and habitat use by Myotis daubentonii and Myotis nattereri during the swarming season: implications for conservation. Animal Conservation 6, 283–290 (2003).
Rivers, N. M., Butlin, R. K. & Altringham, J. D. Autumn swarming behaviour of Natterer’s bats in the UK: population size, catchment area and dispersal. Biological Conservation 127(2), 215–226 (2006).
Kohyt, J., Rozik, A., Kozakiewicz, K., Pereswiet-Soltan, A. & Gubala, W. J. Activity pattern and fat accumulation strategy of the Natterer’s bat (Vespertilionidae, Chiroptera) swarming population indicate the exact time of male mating effort. Mammal. Research 61, 383–389 (2016).
Parsons, K. N., Jones, G., Davidson-Watts, I. & Greenaway, F. Swarming of bats at underground sites in Britain – implications for conservation. Biological Conservation 111, 63–70 (2003).
Glover, A. M. & Altringham, J. D. Cave selection and use by swarming bat species. Biological Conservation 141, 1493–1504 (2008).
Piksa, K., Bogdanowicz, W. & Tereba, A. Swarming of bats at different elevations in the Carpathian Mountains. Acta Chiropterologica 13(1), 113–122 (2011).
Encarnação, J. A. Mating at summer sites: indications from parentage analysis and roosting behaviour of Daubenton’s bats (Myotis daubentonii). Conservation Genetics 13, 1161–1165 (2012).
Beasley, L. J. & Zucker, I. Photoperiod influences the annual reproductive cycle of the male pallid bat (Antrozous pallidus). Journal of Reproductive Fertility 70, 567–573 (1984).
Kunz, T. H., Wrazen, J. A. & Burnett, C. D. Changes in body mass and fat reserves in pre-hibernating little brown bats (Myotis lucifugus). Ecoscience 5(1), 8–17 (1998).
Culina, A., Linton, D. M. & Macdonald, D. W. Age, sex and climate factors show different effects on survival of three different bat species in a woodland bat community. Global Ecology and Conservation 12, 263–271 (2017).
McGuire, L. P. et al. Common condition indices are no more effective than body mass for estimating fat stores in insectivorous bats. Journal of Mammalogy 99(5), 1065–1071 (2018).
Gaisler, J. & Zukal, J. Ecomorphometry of Myotis daubentonii and M. lucifugus (Chiroptera, Vespertilionidae) – a Palearctic-Nearctic comparison. Mammalia 86(4), 275–282 (2004).
Culina, A., Linton, D. M., Pradel, R., Bouwhuis, S. & Macdonald, D. W. Live fast, don’t die young: Survival-reprodution trade-offs in long-lived income breeders. Journal of Animal Ecology 88, 746–756 (2019).
Norquay, K. J. O. & Willis, C. K. R. Hibernation phenology of Myotis lucifugus. Journal of Zoology 294(2), 85–92 (2014).
Czenze, Z. J., Jonasson, K. A. & Willis, C. K. R. Thrifty Females, Frisky Males: Winter Energetics of Hibernating Bats from a Cold Climate. Physiological and Biochemical Zoology 90(4), 502–511 (2017).
Parsons, K. N. Autumnal swarming of bats (Chiroptera) in Britain. Unpublished doctoral thesis, University of Bristol (2003).
Hoying, K. M. & Kunz, T. H. Variation in size at birth and post-natal growth in the insectivorous bat Pipistrellus subflavus (Chiroptera: Vespertilionidae). Journal of Zoology 245, 15–27 (1998).
Racey, P. A. Ageing and assessment of reproductive status of pipistrelle bats, Pipistrellus pipistrellus. Journal of Zoology 173(2), 264–271 (1974).
Adams, R. A. Bat reproduction declines when conditions mimic climate change projections for western North America. Ecology 91, 2437–2445 (2010).
Bates, D., Maechler, M., Bolker, B. & Walker, S. Fitting linear mixed-effects models using lme4. Journal of Statistical Software 67(1), 1–48 (2015).
R Core Team. R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria, https://www.R-project.org/ (2019).
Christensen, R.H.B. Ordinal – Regression Models for Ordinal Data. R package version 2015.6-28, http://www.cran.r-project.org/package=ordinal/ (2015).
Mazerolle, M. J. AICcmodavg: Model selection and multimodel inference based on (Q)AIC(c). R package version 2.0-4, http://CRAN.R-project.org/package=AICcmodavg (2016).
Burnham, K. P. & Anderson, D. R. Model Selection and Multimodel Inference: A Practical Information-Theoretic Approach (2nd Edition). Springer, New York (2002).
Acknowledgements
The authors would like to thank all the citizen scientists who assisted with data collection. Professor Fiona Mathews for enabling DML to act as an accredited agent on Natural England Project Licence 2018-36143-SCI-SCI and preceding licences. Nigel Fisher, Conservator of Wytham Woods, and his staff for their help and support. The Edward Grey Institute (EGI) researchers and field assistants for their collaboration and access to equipment. Dr Paul Johnson (WildCRU) for modelling advice. This work was generously supported by a grant to DWM from S. Durie, with additional support from the Peoples’ Trust for Endangered Species.
Author information
Authors and Affiliations
Contributions
D.M.L. conceived the project aims and objectives; D.M.L. and D.W.M. agreed the methodology; D.M.L. collected and analysed the data, and wrote the manuscript. D.W.M. contributed critically to drafts and gave final approval for publication.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Linton, D.M., Macdonald, D.W. Phenology of reproductive condition varies with age and spring weather conditions in male Myotis daubentonii and M. nattereri (Chiroptera: Vespertilionidae). Sci Rep 10, 6664 (2020). https://doi.org/10.1038/s41598-020-63538-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-020-63538-y
This article is cited by
-
Long-term field study reveals that warmer summers lead to larger and longer-lived females only in northern populations of Natterer’s bats
Oecologia (2023)
-
Energy allocation shifts from sperm production to self-maintenance at low temperatures in male bats
Scientific Reports (2022)
-
Long-term field studies in bat research: importance for basic and applied research questions in animal behavior
Behavioral Ecology and Sociobiology (2022)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.